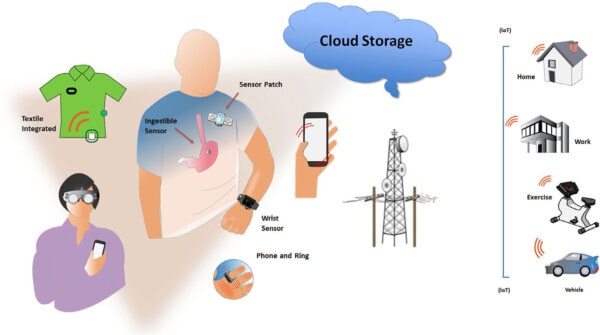
January 29, 2021—This ISHNE/HRS/EHRA/APHRS collaborative statement describes the current status of mobile health (mHealth) technologies in arrhythmia management. The range of digital medical tools and heart rhythm disorders that they may be applied to and clinical decisions that may be enabled are discussed. The facilitation of comorbidity and lifestyle management (increasingly recognized to play a role in heart rhythm disorders) and patient self-management are novel aspects of mHealth. The promises of predictive analytics but also operational challenges in embedding mHealth into routine clinical care are explored.
The aim of this document is to define state-of-the-art mHealth technologies and their application in arrhythmia management and explore future directions for clinical application. As such, the scope of the document encompasses discussion of the different mHealth technologies currently available or in development; the acquisition of health-related data; the applications of such data, including disease identification and management; clinical trials; the patient perspective; and the issues that must be addressed in the future to permit useful application of mHealth technologies. Additionally, discussion is extended to mHealth facilitation of those comorbidities increasingly recognized to influence arrhythmia management (e.g., obesity and sleep apnea) that are becoming the responsibility of heart rhythm professionals.
The Russian and Spanish (Executive Summary) translations are available.
Topic
- Atrial Fibrillation
- Clinical EP
- Clinical Topics
Resource Type
- Clinical Documents
Related Resources

Clinical Documents
18F-FDG PET/CT and Radiolabeled Leukocyte SPECT/CT Imaging for the Evaluation of Cardiovascular Infection in the Multimodality Context
March 11, 2024

Clinical Documents
2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Patients With Atrial Fibrillation
November 30, 2023

Clinical Documents
2003 NASPE Training Requirements for Cardiac Implantable Electronic Devices: Selection, Implantation, and Follow-Up
July 1, 2023