December 17, 2024 – Boston Scientific has identified a subpopulation of dual chamber, standard life and extended life pacemakers; cardiac resynchronization therapy pacemakers (CRT-Ps) with an increased potential to initiate Safety Mode during telemetry or other normal, higher-power operations due to high battery impedance.
Authors
Molly Sachdev, MD; Amit J. Shanker, MD; Lisa Miller, MS; Anne Marie Smith, MBA; Kenneth A. Ellenbogen, MD; Christopher F. Liu, MD
Correspondence
[email protected]
Impacted Models
ACCOLADE™, PROPONENT™, ESSENTIO™, and ALTRUA™ 2 dual chamber (DR) standard life (SL) and DR extended life (EL) pacemakers; and VISIONIST™ and VALITUDE™ cardiac resynchronization therapy pacemakers (CRT-Ps) built before Sep 2018. For specific model numbers, please reference the Dear Doctor letter provided by Boston Scientific, your field representative, technical services, and/or the device look-up tool www.BostonScientific.com/lookup.
Recall Issue Summary
A subset of product manufacturers who built devices before Sep 2018 used battery cathode processing techniques that resulted in higher concentration of lithium salts, which can be associated with high battery impedance later in device life. This results in an increased potential to initiate Safety Mode during telemetry or other normal, higher-power operations towards the end of device life. The non-programmable Safety Mode pacing parameters may not provide optimal support of a patient’s cardiac condition.
In certain patients, Safety Mode may result in pacing inhibition/pauses, muscle stimulation (e.g., skeletal muscle or phrenic nerve stimulation), or heart failure decompensation prior to device replacement. The worst-case reported patient harm has been loss of pacing with serious injury or life-threatening outcome. There have been two (2) deaths in pacemaker dependent patients after initiating Safety Mode in an ambulatory setting. The table below shows the estimated incidence of such malfunction: (for comparison, estimated incidence of malfunction in the non-advisory population is 0.1% at 9 years).
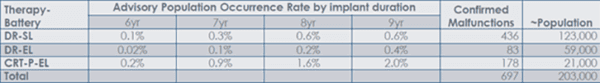
Because the advisory devices were built before Sep 2018, there are no remaining devices within the advisory population available for implantation.
In comparison to the prior 2021 Boston Scientific advisory on different lines of pacemakers also reaching Safety Mode (last updated in 2023), the rates of malfunction in this current advisory are lower.
Manufacturer Recommendations
- Individual patient evaluation. Promptly identify patients within the advisory population who are at risk of harm due the non-programmable parameters in Safety Mode.
- Replacement– Replace all devices that have entered Safety mode, emergently for patients at risk of harm, and urgently for all others.
Note: During replacement of a device in Safety Mode, pacing inhibition should be anticipated during electrocautery and when the device is removed from the pocket due to unipolar pacing and high sensitivity.- Prophylactic generator replacement is recommended for patients with a device from the advisory population AND who are at risk of harm due to non-programmable parameters in Safety Mode (ie, pacing-dependent), when the longevity remaining reaches four (4) years or if the longevity remaining is already less than 4 years. - Monitoring– Recommended follow-up intervals (for in-person and remote) remain unchanged in accordance with the original instructions for use.- For patients who are recommended for prophylactic replacement: There is a potential for pacing pauses during in-person checks and LATITUDE patient-initiated interrogation (PII). During in-person device checks for such patients in the advisory population, consider patient recumbency and availability of resuscitation equipment with qualified personnel. Consider disabling PII for such patients on LATITUDE.Boston Scientific is actively developing a software update for the advisory devices designed to detect the onset of a high impedance battery state and display a device-based alert via the LATITUDE™ programmer and remote patient management system prior to Safety Mode initiation.
HRS Recommendations
HRS strongly encourages its members worldwide to read the detailed Dear Doctor letter provided by Boston Scientific.
We agree that prophylactic generator change should be immediately performed in patients who have either already entered Safety Mode, or those in the advisory population who are at risk of serious harm if they enter Safety Mode (pacing-dependent patients). In the latter case, the generator should be changed at 4 years of remaining battery longevity as per manufacturer recommendation, or timing can be discussed using an informed decision-making approach. Prophylactic generator change is not recommended for the non-advisory population or for advisory population patients felt to be NOT at risk of harm in case of Safety Mode.
Reporting Contact
Adverse events should be reported to your local Boston Scientific representative or Technical Services and to regulatory authorities such as the FDA for our US based members.
FDA’s MedWatch
Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
If further support is required, contact Boston Scientific Technical Services at 1-800-227-3422.
Topic
- Regulatory
- Regulatory Agencies
- U.S. Food and Drug Administration (FDA)
Resource Type
- Safety Alerts
Manufacturer
- Boston Scientific
Device Type
- CRT-Ps
- Pacemakers
FDA Class
- Class II
Related Resources

Safety Alerts
Biosense Webster Advisory: Update to the Instructions for Use for VARIPULSE
March 26, 2025

Safety Alerts
Abbott Advisory: Manufacturing Defect Associated with Loss of Pacing for a Limited Subset of Assurity™ and Endurity™ Pacemakers
February 18, 2025

Safety Alerts
Philips Urgent Medical Device Correction: Mobile Cardiac Telemetry (MCT) or Event Diagnostic Service Processing
February 5, 2025